The National Health Service (NHS) has approved two cannabis-based medicines used to treat epilepsy and multiple sclerosis.
The decision follows new guidelines from the National Institute for Clinical Excellence (NICE), a drugs advisory body.
Doctors will be able to prescribe Epidyolex for children with two types of severe epilepsy – Lennox Gastaut syndrome and Dravet syndrome.
Clinical trials have shown the oral solution, which contains cannabidiol (CBD), could reduce the number of seizures by up to 40% in some children.
The drug does not contain the main psychoactive component of cannabis, THC.
The lack of medication containing THC has sparked a mixed reaction to the NICE announcement.
The change is “great news”, says Professor Helen Cross, a consultant in paediatric neurology at Great Ormond Street Hospital who led UK trials of Epidyolex.
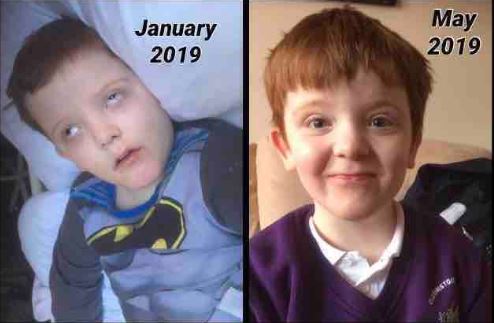
She told the BBC: “Dravet and Lennox Gastaut syndromes are both complex difficult epilepsies with limited effective treatment options and this gives patients another option… that could make a difference to care.”
And Galia Wilson, chairwoman of Dravet Syndrome UK, said: “Many families come to us asking about the potential of cannabis-based medicines, particularly cannabidiol, and we are thrilled that one is now available on the NHS.”
However, the change has been slammed by a Scottish mum whose son has severe epilepsy.
Karen Gray, from Edinburgh, says her son Murray went from 600 seizures a day to none in more than 140 days since she started administering cannabis oil with THC.
Murray has from a rare form of epilepsy called Doose Syndrome, but Karen is forced to pay around £1,200 a month to buy the oil from Holland.
She said: “Epidyolex doesn’t help Murray. It’s ridiculous for the many people using THC for pain.
“We’re trying to get THC. We are all very disappointed.”
The campaign group, End Our Pain, said the new guidelines were a “massive missed opportunity”.
Spokeswoman Millie Hinton said: “This restrictive guidance is condemning many patients to having to pay for life-transforming medicine privately, to go without or to consider accessing illegal and unregulated sources.”


